Note: This lesson deals with compounds made up of atoms from two elements. When you have atoms of more than two elements in a compound, you cannot use electronegativity differences to predict whether the compound is polar. Instead, you have to look at the molecular geometry to determine the compound’s polarity.
Have students conduct a think-pair-share activity, using this prompt: “Real, three-dimensional atoms are very different from the Lewis dot structures we have been using. How are Lewis dot structures helpful to us?” (Discussion should focus on the fact that Lewis dot structures show valence electrons, so that we can show how and why atoms bond together.)
Tell students the objectives for this lesson. Explain that when all the atoms in a covalent molecule are identical the attraction each atom has for shared electrons is equal. “What is the measure of an atom’s attraction for electrons called?” (electronegativity) When the atoms in a covalent molecule are different, the attraction for the shared electrons is not equal. In a covalent molecule such as Cl2, the attraction for the shared electrons is equal because the two chlorine atoms are the same. A molecule of HCl is also covalently bonded, but chlorine has a greater attraction for the electrons than hydrogen does. Diagram both of these on the board; define and label them “polar” and “nonpolar.” Have students copy the diagrams in their notes.
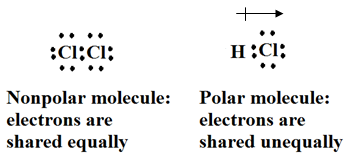
Explain that the symbol  is used in diagrams of polar covalent molecules, with the arrow pointing in the direction of the atom that has a greater pull on the electrons. The electrons spend more time near the chlorine atom, so it has a partial negative charge and less time near the hydrogen atom, so it has a partial positive charge.
is used in diagrams of polar covalent molecules, with the arrow pointing in the direction of the atom that has a greater pull on the electrons. The electrons spend more time near the chlorine atom, so it has a partial negative charge and less time near the hydrogen atom, so it has a partial positive charge.
Ask, “Which of the following molecules with covalent bonds should have atoms with equal attraction for electrons? H2, F2, HF, NH3.” (H2 and F2 have identical atoms that equally share electrons.)
Define diatomic molecule and explain that O2 and I2 are other examples of diatomic molecules.
Ask students to take out their periodic tables and electronegativity trends document. Ask, “How can we use electronegativity to predict which type of bond will form between atoms?” (We can find the difference in the atoms’ electronegativity values; those with a difference greater than 1.7 form ionic bonds, and those with a difference equal to or less than 1.7 form covalent bonds.)
Have students copy the following chart into their notes:
|
Difference in Electronegativity
|
Type of Bond
|
|
<0.5
|
Nonpolar covalent
|
|
0.5–1.7
|
Polar covalent
|
|
˃1.7
|
Ionic
|
Draw the following diagrams of HBr (hydrogen bromide) on the board and ask which correctly shows the partial charges. (The diagram on the right is correct.)
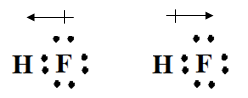
Have students practice by identifying which of the following elements will form ionic, polar covalent, and nonpolar covalent bonds together, based on their electronegativity differences: CO, I2, HBr, MgF. (CO is polar covalent, I2 is nonpolar covalent, HBr is polar covalent, and MgF is ionic.)
Explain that electronegativity differences can also help explain differences in the boiling points of different molecules. In the polar molecule HCl, partial charges hold the molecule together (shown above). Ask, “What is the electronegativity difference between H and Cl?” (0.9) In the ionic compound NaCl, the difference is greater at 2.1. This means that the atoms are held together more tightly in NaCl than in HCl, so NaCl has a higher boiling point. Have students write in their notes:
- Greater electronegativity difference = higher boiling point.
- Ionic compounds have higher boiling points than covalent molecules.
Define dipole as the direction of a positive and negative partial charge produced in a polar covalent molecule. Use the diagram of HBr to show how the electrons spend more time near the fluorine atom, which creates a dipole.
Conclude the lesson by having students write definitions in their own words for the following terms, or assign as homework: polar covalent, nonpolar covalent, diatomic molecule, dipole.
Day 2
Review content from Day 1 using the Polar and Nonpolar Covalent Bonding handout, which shows more examples of how electrons are shared in polar and nonpolar covalent molecules (S-C-6-2_Polar and Nonpolar Covalent Bonding.doc).
Have students complete the Types of Bonds worksheet (S-C-6-2_Types of Bonds and KEY.doc). Go over the answers with the class, and provide further instruction as necessary.
Have students complete the chart on the worksheet Comparing Ionic, Polar Covalent, and Non-Polar Covalent Bonding (S-C-6-2_Comparing Ionic, Polar Covalent, and Non-Polar Covalent Bonding and KEY.doc). If time is limited, assign this worksheet for homework. Collect and check the worksheets for assessment of students’ understanding.
Extension:
- Tailor the content for students who might need an opportunity for additional learning by providing additional examples and having students practice drawing Lewis dot structures for ionic, polar covalent, and nonpolar covalent bonds.
- Students who may be going beyond the standards can research how polarity explains why oil and water don’t mix, and prepare a mini-poster with a diagram of oil and water molecules. The mini-poster should show the partial charges on polar water molecules attracting each other, pushing nonpolar oil molecules out of the way. It should also explain that surface tension in water is due to the attraction of the partial charges on the hydrogen and oxygen atoms between the water molecules.
