To review background knowledge, this activity asks students to build models of ionic and covalent bonds between atoms. Students need to understand the parts of an atom prior to this lesson. Students also need a basic understanding of the periodic table of the elements. Students should also understand how to write basic chemical equations.
In advance of the lesson, prepare paper bags of materials for the Day 3 lab. Examples of common ionic bonds include NaCl, MgCl, LiF, KBr, LiCl, MgO, Li2O, and AlCl3. Examples of common covalent bonds include H2O, CH4, CO2, NH3, O2, H2, HCl, and CCl4. Read through the lab and practice demonstrating it.
Day 1
To introduce the lesson, have students answer the following prompt in their science notebooks. “Diagram the basic structure of an atom and label the atom’s protons, neutrons, and electrons. Write a definition for atom and molecule under the diagram.” Allow several minutes for independent work. Then, as a class, agree upon definitions for atom and molecule. Write the definitions on the board for students to copy in their notes.
Tell students the objectives for this lesson. Have students take out their periodic table handouts (S-C-6-1_Periodic Table.pdf).
Ask, “How can we tell how many electrons are in an atom of an element?” (The atomic number is equal to the number of electrons.) Ask, “How many electrons does an atom of oxygen have?” (8) “…hydrogen? Magnesium?” (Hydrogen has 1 electron, and magnesium has 12 electrons.)
Explain that when atoms combine, only the electrons in the outermost energy level are involved. These are the valence electrons. Show students how to use the group number of an element to determine the number of valence electrons. For example, ask them how many electrons a carbon atom has (6), and then tell them that there are two electrons in the first energy level and four valence electrons.
Tell students that the number of valence electrons is equal to the group number for representative elements. For example, hydrogen is in group 1 and it has 1 valence electron. Neon is in group 18 and it has 8 valence electrons. The only exception is helium, which is in group 18 but only has 2 valence electrons. Check for understanding by asking students the number of valence electrons for several other elements, such as calcium, boron, and silicon. (Calcium has 2, boron has 3, and silicon has 6 valence electrons.)
Tell students that we can use a diagram called a Lewis dot structure to draw an atom with its valence electrons. On the board, draw the Lewis dot structures of a carbon atom and a magnesium atom, as shown below.
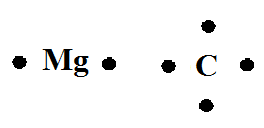
Hand out Valence Electrons and Lewis Dot Structures (S-C-6-1_Valence Electrons and Lewis Dot Structures and KEY.doc). Have students complete Parts 1 and 2. It may be helpful to model an example for each part before students begin working. Go over the answers with the class.
Tell students that Lewis dot structures can be used to show how the arrangement of electrons changes when atoms combine to form compounds.
Explain the octet rule, that states that atoms tend to combine in such a way that they each have eight electrons in the highest main energy level, giving them the same electronic configuration as a noble gas. It is also called the noble gas rule. The noble gases do not react with other elements. They are very stable because the s- and p- sublevels in the highest main energy level are filled. Noble gases have 8 electrons in their outer shell (except helium, which has 2).
Guide students step-by-step in completing the Octet Rule Chart (S-C-6-1_Octet Rule Chart and KEY.doc).
Wrap up the lesson by asking students to explain why atoms combine with one another. (They combine in order to have a stable valence electron shell.)
Day 2: Trends in the Periodic Table.
Have students take out their periodic tables. Tell them that we can use the periodic table to predict which elements are more likely to bond with one another.
Explain that there are two types of bonds, ionic and covalent. For now, just tell students that atoms in covalent bonds share electrons and atoms in ionic bonds transfer electrons. Show students a picture of covalent and ionic bonds (S-C-6-1_Covalent and Ionic Bonds.doc). Point out to students that ionic bonding occurs between metals and nonmetals, and covalent bonding occurs between two nonmetals. For example, Na is a metal and Cl is a nonmetal, so NaCl will form an ionic bond.
Define ions as atoms that have acquired an electrical charge by either gaining or losing electrons. Explain that metals tend to lose electrons, forming positive ions (cations). Nonmetals tend to gain electrons, forming negative ions (anions). If they have not already done so, guide students in labeling or color-coding the metals and nonmetals on their periodic tables. Have students identify the following compounds as ionic or covalent:
MgO (ionic)
CaCl2 (ionic)
SO2 (covalent)
PbCl2 (ionic)
CCl4 (covalent)
CH4 (covalent)
Next, explain the concept of electronegativity, which is a number that describes the ability of an atom to attract electrons when it forms a bond with another atom. Give students copies of Electronegativity Trends in the Periodic Table (S-C-6-1_Electronegativity Trends.doc). Have them look at the trends on the periodic table and add this sentence below it, “Electronegativity increases from left to right, and decreases from top to bottom.” Ask, “Why don’t most of the noble gases have electronegativity values?” (Because they do not have valence electrons that are available to form chemical bonds.)
Tell students we can determine the type of bond based on the difference in electronegativity between atoms. Have them copy the following notes on the handout:
- Difference in electronegativity (ΔEN) = higher EN – lower EN
- ΔEN less than 1.7 = covalent bond
- ΔEN more than 1.7 = ionic bond
Example 1: HCl
ΔEN = 3.0 – 2.1 = 0.9
Example 2: NBr3
ΔEN = 3.0 – 2.8 = 0.2 (for each bond)
Have students determine the ΔEN and type of bond for these compounds: CrO, Br2, CH4, and KCl.
Solutions:
ΔEN of CrO = 3.5 − 1.6 = 1.9 (ionic)
ΔEN of Br2 = 2.8 − 2.8 = 0 (covalent)
ΔEN of CH4 = 2.5 − 2.1 = 0.4 (covalent)
ΔEN of KCl = 3.0 − 0.8 = 2.2 (ionic)
Ionic Bonding
Explain how the octet rule relates to ions. Define ions as atoms that have acquired an electrical charge by either gaining or losing electrons. Tell students that a neutral atom has a number of electrons equal to the number of protons (e.g., a sodium atom that has 11 electrons). Have them write down the definitions of ion and neutral atom. Have them recall the Lewis dot structure for sodium (from the worksheet), and ask how many valence electrons it has. (one)
Tell students sodium will lose one electron to attain a noble gas configuration. Chlorine will take the electron and leave sodium with the noble gas configuration. When sodium and chlorine bond together, sodium becomes an ion (Na+), because it has lost one electron. Have students copy the following notes; explain the diagrams as you present them. Point out that the metal is listed first, and then the nonmetal.
sodium + chlorine → sodium chloride
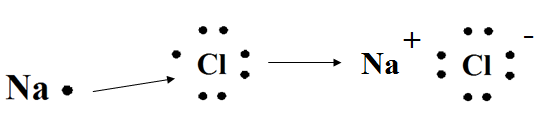
Explain that when Na combines with Cl, the Na becomes a cation (positive ion), and the Cl becomes an anion (negative ion). If needed, review how to use the octet rule to find the ionic charge for atoms of various elements from Day 1.
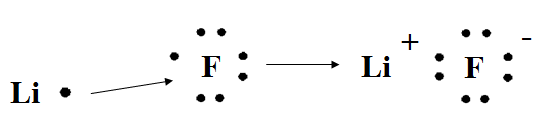
Have students practice by drawing Lewis dot structures to show how a bond forms between Li and F.
lithium + fluorine → lithium fluoride
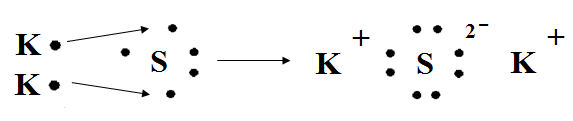
Give students an example of a more complex ionic bond, shown below. Explain that atoms will follow the octet rule when they form bonds, so that their valence shells are full. It may require several atoms to fulfill the octet rule.
potassium + sulfur → potassium sulfide
Give students the Ionic Bonding worksheet (S-C-6-1_Ionic Bonding and KEY.doc). Have them complete the worksheet individually or in pairs, and then go over the answers with the class. Provide more examples of ionic bonding and ask questions about them to assess students’ understanding of the concept. Have students copy the diagrams into their notes.
Covalent Bonding
Remind students that covalent bonding occurs between two nonmetals. In covalent bonding, electrons are shared instead of transferred. Covalent bonding follows the octet rule like ionic bonding does.
Show students the covalent bonding between hydrogen and fluorine using the diagrams below. Explain the electron shell diagram and the Lewis dot structure as you draw them on the board and have students copy them into their notes.

Give students the Covalent Bonding worksheet (S-C-6-1_Covalent Bonding and KEY.doc). Guide students through the worksheet examples, and have them complete the problems. Go over the answers with the class. If necessary, provide more examples of multiple bonds, such as Br2 and O2.
Have students copy the following guidelines for drawing Lewis dot structures into their notes:
1. Count the total number of valence electrons.
2. Draw a skeleton of the molecule (just the atomic symbols).
3. Draw the bonds; each bond is made up of two electrons.
4. Draw in the remaining electrons.
5. Check the octet rule for each atom.
6. If needed, make double or triple bonds to fulfill the octet rule.
Day 3
Lab: Modeling Ionic and Covalent Bonds
Hand out the worksheet Ionic versus Covalent Bonding (S-C-6-1_Ionic versus Covalent Bonding and KEY.doc). Have students complete the worksheet before beginning the lab.
Explain to students that they will demonstrate their understanding of ionic and covalent bonds by creating models of them. Divide students into pairs, and give each pair a bag containing the gumdrops and bamboo skewer pieces. Use the sample bag of materials to explain the purpose of the different materials by creating a model of sodium chloride (NaCl) as an example. Each large gumdrop represents the nucleus of an atom. The small gumdrops represent electrons. Students should note that there are two colors of gumdrops, and each atom to be created should use one color; for example, if creating sodium chloride, the electrons in the sodium atom should be one color and the electrons in the chlorine atom should be the other color. The different lengths of bamboo skewers will help students create the different energy levels of electrons.
Have each pair of students draw a slip of paper from the bag containing simple ionic bonds, and draw a slip from the bag containing simple covalent bonds. Students should use the materials in their bags to create models of the ionic and covalent bonds they have selected. Tell students that depending on the bonds they are creating, they may not use all of the materials. When pairs finish a model, have them raise their hands to notify you that they are ready for you to check the model. Once you approve a model, the pair should tape the slip of paper with the type of bond to one of the skewers. If time permits, these slips can be removed and students can use their knowledge of the elements to determine what molecules and types of bonds are being represented.
To close the lesson, have each student diagram three-dimensional models of ionic and covalent bonds, labeling each atom and the type of molecule that is shown.
Extension:
- Students who might need an opportunity for additional learning can color-code the electrons from different elements in a compound. For example, in H2O, the hydrogen electrons may be black and the oxygen electrons may be red. It may be helpful to have students manipulate coins or other objects to represent the sharing or transfer of electrons in ionic and covalent bonding. Provide students with additional examples of ionic and covalent bonding, and allow extra time for reviewing the practice problems step-by-step.
- Students who may be going beyond the standards can read the article, “Glue of Molecular Existence Is Finally Unveiled” found at www.nytimes.com/learning/teachers/featured_articles/19990907tuesday.html. Then have them write a paragraph to summarize the article.
- During the lab, students who may be going beyond the standards can create models of more complex molecules, such as O3 (ozone), Al2O3 (aluminum oxide), or H2SO4 (sulfuric acid).