Have students take out their science
notebooks. Tell them that you are going to show two pictures and
perform one demonstration. They are to answer one simple question
regarding all three things at the end. Show students the following
pictures, or similar pictures, and ask the corresponding questions
(S-C-3-1_Gas Tank and Rust Pictures.doc).
Pour 10 ml of vinegar into a resealable
plastic bag. Add 1 spoonful of baking soda to the bag. Close the bag
immediately. Tell students what the reactants are as you add them to
the bag. Wait for the bag to expand. Ask students, “Where did
the gas in the bag come from? Where did the baking soda go?”
Allow students a few minutes to respond to
the questions by writing in their notebooks. When students have
completed their responses, ask for a few sample responses to each
question. Then respond by saying, “The gasoline that you put in
your car seems to disappear. The rust seems to appear from nowhere.
The baking soda disappears, while a gas develops. These are all
examples of chemical reactions. In the demonstration, the baking soda
chemically reacts with the vinegar to produce carbon dioxide gas.
That is why we saw the bag inflate. The atoms that make up the baking
soda were not lost, nor were the atoms that make up the carbon
dioxide gas created. In fact the atoms in baking soda (NaHCO3)
are rearranged to produce carbon dioxide (CO2)
and other products.” Explain that this is a closed system, in
which the reactants and products are all within the sealed bag. If we
measure the total mass of the reactants and the products, they will
be the same. In an open system (i.e., open container), the CO2
gas would have dispersed in the surrounding environment.
The reaction (two-step) is as follows:
(1) CH3COOH +
NaHCO3 H2CO3
+ NaC2H3O2
(2) H2CO3
CO2 + H2O
Note: The last product of the first
reaction is carbonic acid, which quickly decomposes into carbon
dioxide and water. The CO2 is what you see foaming and
bubbling in this reaction. Vinegar is an aqueous solution of acetic
acid.
Continue by saying, “As you can see,
the atoms are not lost as the reaction proceeds nor are atoms
created. They are merely rearranged. Let’s investigate why this is
true and how chemists express this as they write chemical equations.”
“Let’s take a closer look at the
reaction we just talked about. Look at the first reaction between
vinegar and baking soda (sodium bicarbonate). Count the number of
atoms of each element on the reactant side and then on the product
side.” You may need to remind them that the reactants
are on the left side of the arrow and that the products are on the
right side. Guide them in forming lists like these:
|
Reactant Side
|
Product Side
|
|
H = 5
|
H = 5
|
|
O = 5
|
O = 5
|
|
C = 3
|
C = 3
|
|
Na = 1
|
Na = 1
|
Tell them to do the same thing for the
second reaction. Their atom inventory should look like this:
|
Reactant Side
|
Product Side
|
|
H = 2
|
H = 2
|
|
O = 3
|
O = 3
|
|
C = 1
|
C = 1
|
At this point, say, “What do you notice about the number of
atoms on the reactant and product sides of both reactions?”
Students should recognize that the number of atoms of each element
is the same. If students are not getting their atom inventory to
match yours, remind them that the subscripts in the equation
represent the number of atoms for the element before it only. For
example, HCO3 has 1 hydrogen atom, 1 carbon atom and 3
oxygen atoms. Chemists do not write 1 as a coefficient.. Say, “The
fact that atoms are neither created nor destroyed in a chemical
reaction is called the law of conservation of matter. There
are similar laws as well that you may be familiar with such as the
law of conservation of energy and the law of conservation of mass.”
If there are still questions, put an example on the board that has
1:1 molar ratios, such as:
C + O2
CO2 or NaCl + K
KCl + Na
Optionally, give students colored jelly beans in a small cup with
toothpicks. Designate a specific color for an element, such as red
jelly beans for carbon and green jelly beans for oxygen. Have them
construct models of the examples you did from the lesson. Remind
them that they cannot add individual jellybeans, as that would be
analogous to adding a subscript. They may only add complete sets
(molecules/compounds) to satisfy the law of conservation of matter.
After you have formatively assessed that
students are ready to move on (through interaction with the class and
examples on the board), give them a more difficult problem. Say, “I
want you to complete the same task but for a slightly more
challenging reaction. Create an atom inventory
or a list of the number of each atom on both sides of the equation.”
Use the following reaction:
AlCl3 + Na
Al + NaCl
|
Reactant Side
|
Product Side
|
|
Al = 1
|
Al = 1
|
|
Cl = 3
|
Cl = 1
|
|
Na = 1
|
Na = 1
|
Ask students what they notice about the atom
inventory. Was the law of conservation of matter satisfied? (No).
Tell students, “According to the equation as it is currently
written, 2 chlorine atoms seemed to have disappeared as the reaction
progressed. Remember that matter cannot be destroyed, according to
the law of conservation of matter. Chemists use something
called coefficients to address this discrepancy. Coefficients are
large numbers that are placed in front of a molecule, element, or
compound to satisfy the conservation law. After coefficients are used
and there are equal numbers of atoms of each element on both sides of
the equation, it is said to be a balanced equation.”
Say, “If we put a large 3 (a
coefficient) in front of the NaCl, the equation would look like this”
(write on the board):
AlCl3 + Na
Al + 3NaCl
“If you were to create an atom
inventory (count of the number of atoms of each element for both the
reactant and product side), you will see that it now looks like this”
(write on the board):
|
Reactant Side
|
Product Side
|
|
Al = 1
|
Al = 1
|
|
Cl = 3
|
Cl = 1
|
|
Na = 1
|
Na = 3
|
“Notice that the coefficient of 3 is
placed in front of the NaCl. The 3 is distributed to all atoms in the
compound (both the Na and Cl), which fixes the chlorine discrepancy,
but creates a new discrepancy for sodium. It would seem easier to put
the coefficient in the middle of the NaCl like this: Na3Cl, but that
is not allowed. The ionic compound NaCl must be written that way due
to the ionic bond that sodium and chlorine make. The coefficients
cannot be sandwiched between two atoms in a compound. The fact that
chlorine was ‘fixed’ but now sodium is wrong is not a problem.
Another coefficient will be placed like this” (write on the
board):
AlCl3 + 3Na
Al + 3NaCl
“If you complete an atom inventory of
the reaction, it should look like this” (write on the board):
|
Reactant Side
|
Product Side
|
|
Al = 1
|
Al = 1
|
|
Cl = 3
|
Cl = 3
|
|
Na = 3
|
Na = 3
|
“The law of conservation of matter has
been satisfied and the equation is said to be balanced.” At
this point, some students may ask what the coefficients mean and why
chemists can just add them. It may help to compare a chemical
equation to a recipe. Say, “Suppose you had the following recipe
to make a s’more” (write on the board):
1 s’more (S)
2 graham crackers (G)
1 marshmallow (M)
1 piece of chocolate (C)
“If you were to write this recipe out
as if it were a chemical reaction it may look something like this”
(write on the board):
2 G + 1M + 1C
1S
“Notice how you would need two crackers
to make one s’more? If you had only one cracker and you were
following the recipe, you would not be able to make a complete
s’more. Balancing a chemical reaction is similar in that reactions
need specific ratios of reactants in order to occur.” Do a few
more examples on the board.
Example 1
Unbalanced: K + Zn(NO3)2
KNO3 + Zn
|
Reactant Side
|
Product Side
|
|
K = 1
|
K = 1
|
|
Zn = 1
|
Zn = 1
|
|
N = 2
|
N = 1
|
|
O = 6
|
O = 3
|
“The 2 coefficient is applied to both
the potassium and the nitrate ion.”
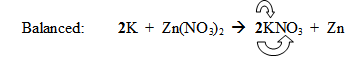
|
Reactant Side
|
Product Side
|
|
K = 2
|
K = 2
|
|
Zn = 1
|
Zn = 1
|
|
N = 2
|
N = 2
|
|
O = 6
|
O = 6
|
Example 2
Unbalanced: Mg(OH)2 + NaCl
MgCl2 + NaOH
|
Reactant Side
|
Product Side
|
|
Mg = 1
|
Mg = 1
|
|
O = 2
|
O = 1
|
|
H = 2
|
H = 1
|
|
Na = 1
|
Na = 1
|
|
Cl = 1
|
Cl = 2
|
Remind students that they cannot add
subscripts. You may need to review how binary ionic compounds are
formed. Some students may want to add a subscript of 2 to the sodium
hydroxide on the product side. Remind them that sodium hydroxide
forms as follows:
Na+1 + OH-1
NaOH
“If you were to change that to Na(OH)2,
you would be indicating that sodium forms a +2 ion, which it does
not.” Therefore, the balanced equation is written as:
Balanced: Mg(OH)2 + 2NaCl
MgCl2 + 2NaOH
Example 3
Unbalanced: CH4 + O2
CO2 + H2O
|
Reactant Side
|
Product Side
|
|
C = 1
|
C = 1
|
|
H = 4
|
H = 2
|
|
O = 2
|
O = 3
|
Tell students, “Note that there is an
odd number of oxygen atoms on the product side. Additionally, the
oxygen atoms on the product side came from two different molecules.
You need to add them together when making your atom inventory.”
Write on the board:
-
Balanced: CH4 + 2O2
CO2 + 2H2O
At this time, you may do more example
problems on the board and/or hand out the Balancing Practice
Worksheet (S-C-3-1_Balancing Practice Worksheet and KEY.doc).
Extension:
-
Students who might need additional practice with balancing
equations can use interactive online activities such as those listed
in Related Resources.
-
Students who may be going beyond the standards can balance
more complex reactions such as the combustion of octane (a component
of gasoline):
-
Unbalanced: C8H18
+ O2 CO2
+ H2O
-
(Note: Balanced:
2C8H18 + 25O2
16CO2 + 18H2O)
