Multiple Choice Items:
-
1. Which of the following is the correct structural formula for C3H8?
-
-
2. Which best describes the formation of a covalent bond?
-
|
A
|
a bond formed through the sharing of inner electrons
|
|
B
|
a bond formed through the sharing of outer electrons
|
|
C
|
a bond formed through the transferring of inner electrons
|
|
D
|
a bond formed through the transferring of outer electrons
|
-
3. Ionic bonds involve which two types of atoms?
-
|
A
|
metals and metals
|
|
B
|
nonmetals and nonmetals
|
|
C
|
nonmetals and metals
|
|
D
|
both A and B
|
-
4. How many valence electrons are in calcium?
-
-
5. The model below shows the structure of a methane molecule. What kind of model is this?
-
-
www.globalwarmingart.com/images/thumb/2/21/Methane_Molecule_VdW.png/250px-Methane_Molecule_VdW.png
-
|
A
|
Lewis dot structure
|
|
B
|
Space-filling model
|
|
C
|
Ball-and-stick model
|
|
D
|
Two-dimensional model
|
-
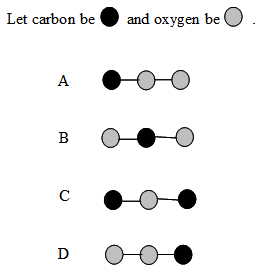
6. Which ball-and-stick model correctly represents CO2?
-
-
7. How many valence electrons are needed for most atoms to have full outer shell?
-
-
8. How many valence electrons are in the following Electron Dot Structure of oxygen?

-
-
9. How many electrons are being shared in the ozone molecule shown here?
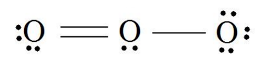
Multiple Choice Answer Key:
|
1. A
|
2. B
|
3. C
|
4. B
|
5. B
|
|
6. B
|
7. C
|
8. B
|
9. B
|
|
Short-answer Items:
-
10. Draw a Lewis dot structure, a ball-and-stick model, and a space-filling molecule for carbon dioxide (CO2).
-
11. Draw a Lewis dot structure, a ball-and-stick model, and a space-filling molecule for diatomic iodine (I2).
Short-answer key and Scoring Rubrics:
-
10. Draw a Lewis dot structure, a ball-and-stick model, and a space-filling molecule for carbon dioxide (CO2).
-
Lewis dot structure:
-
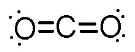
-
-
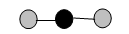
Ball-and-stick model:
-
-
Space-filling model:
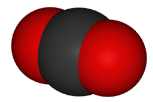
http://www.globalwarmingart.com/wiki/File:Carbon_Dioxide_Molecule_Formula_png
|
Points
|
Description
|
|
3
|
|
|
2
|
Student completes two of the following:
|
|
1
|
Student completes one of the following:
|
|
0
|
Student does not complete any correct drawings or does not answer the question.
|
-
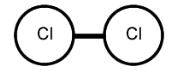
11. Draw a Lewis dot structure, a ball-and-stick model, and a space-filling molecule for diatomic chlorine (Cl2).
-
Lewis dot structure:
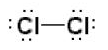
Ball-and-stick model:
Space-filling model:
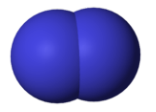
http://upload.wikimedia.org/wikipedia/commons/thumb/6/6c/Nitrogen-3D-vdW.png/150px-Nitrogen-3D-vdW.png
|
Points
|
Description
|
|
3
|
|
|
2
|
Student completes two of the following:
|
|
1
|
Student completes one of the following:
|
|
0
|
Student does not complete any correct drawings or does not answer the question.
|
Performance Assessment:
Construct the following five compounds:
-
MgCl2
-
CO
-
NH3
-
C2H6
-
C2H4
-
Use the following color codes for gum drops and atoms:
Hydrogen = clear or white
Oxygen = red
Nitrogen = yellow
Magnesium = green
Chlorine = blue
You may use your periodic table. First, sketch the molecules on scratch paper. Then construct them using gumdrops to represent atoms and toothpicks to represent bonds.
Place labeled note cards under your models to indicate whether the compounds are ionic or covalent (S-C-4_Performance Assessment Labels.doc).
Performance Assessment Answer KEY and Scoring Rubric
Construct the following five compounds:
Use the following color codes for gum drops and atoms:
Hydrogen = clear or white
Oxygen = red
Nitrogen = yellow
Magnesium = green
Chlorine = blue
Note: This Answer key does not reflect the color code for the assignment. (S-C-4_Performance Assessment Answer KEY.docx).
MgCl2 compound: Ionic
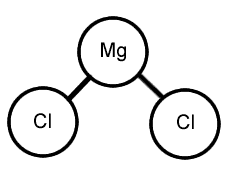
www.cstephenmurray.com/onlinequizes/chemistry/ClassificationOfMatter/AtomsandMolecules/MgCl2.gif
CO molecule: Covalent
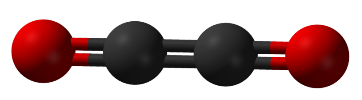
http://upload.wikimedia.org/wikipedia/commons/thumb/3/36/Dicarbon-dioxide-3D-balls.png/800px-Dicarbon-dioxide-3D-balls.png
NH3 molecule: Covalent
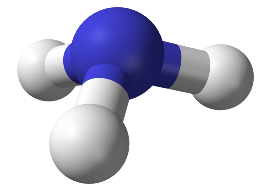
http://upload.wikimedia.org/wikipedia/commons/thumb/0/05/Ammonia-3D-balls-A.png/777px-Ammonia-3D-balls-A.png
C2H6 molecule: Covalent
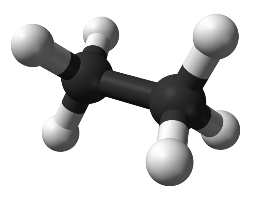
http://upload.wikimedia.org/wikipedia/commons/thumb/3/3c/Ethane-A-3D-balls.png/777px-Ethane-A-3D-balls.png
C2H4 molecule: Covalent
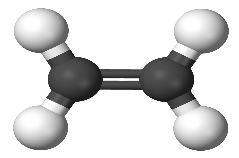
http://upload.wikimedia.org/wikipedia/commons/a/a1/Ethylene-3D-balls.png
Performance Assessment Scoring Rubric:
|
Points
|
Description
|
|
5
|
The student completes all five of the requirements:
-
correctly creates MgCl2 compound and identifies it as an ionic compound.
-
correctly creates CO molecule and identifies it as a covalent molecule.
-
correctly creates NH3 molecule and identifies it as a covalent molecule.
-
correctly creates C2H6 molecule and identifies it as a covalent molecule.
- correctly creates C2H4 molecule and identifies it as a covalent molecule.
|
|
4
|
The student completes four of the five requirements.
|
|
3
|
The student completes three of the five requirements.
|
|
2
|
The student completes two of the five requirements.
|
|
1
|
The student completes one of the five requirements.
|
|
0
|
The student demonstrates lack of understanding or does not attempt to complete the assessment.
|